Samaaro + Your CRM: Zero Integration Fee for Annual Sign-Ups Until 30 June, 2025
- 00Days
- 00Hrs
- 00Min

Pharmaceutical and medical events take place in some of the most highly regulated environments in the world. All programming must ensure that every session, speaker and CME credit issued is compliant, and every touchpoint with a healthcare professional (HCP) must be compliant, traceable, and transparent.
Pharma conferences, CME and other medical programs are, by contrast to almost all corporate events, multi-layered productions. Education, compliance, engagement, and accreditation, all governed by different regulations must be addressed and content must blend all these components together. Using discrete tools to manage each layer creates data silos, reporting mistakes, layers of risk, and ineffectiveness.
For example, a single inconsistency with an attendance log reported to a CME issuing body could lead to disputes or worse, a red flag with a regulator. Many pharma teams are still using spreadsheets, email threads and generating certificates manually, both error-prone when managing hundreds of doctors at large-scale events.
Expectations for the industry have changed and regulators, associations and participants all seek speed, transparency and seamless digital experiences that can be backed up with data. The solution is consolidating compliance, attendee engagement and event management technology together into a single ecosystem.
For a long time, compliance has been an integral part of pharma meetings. However, considering growing scrutiny around the world, compliance is no longer a simply a box to check, compliance represents reputation protection.
Medical event planners are bound by both regional and/or international standards that guide their interactions with healthcare professionals (HCPs). These include declaring sponsorship, accurate allocation of CME credits and retaining genuinely independent educational content.
The Risks of Getting It Wrong:
Example:
A European medical association faced negative feedback after issuing CME certificates to attendees who didn’t fulfil the attendance requirements for the session, a process reliant on manual entry. The overall integrity of the event had been damaged; the association was then tasked with reissuing hundreds of certificates, costing time, and possibly trust, to all involved.
Compliance isn’t a nice-to-have, it’s a foundation. And as events grow and have worldwide participation, continuing to manage compliance elements manually is no longer feasible.
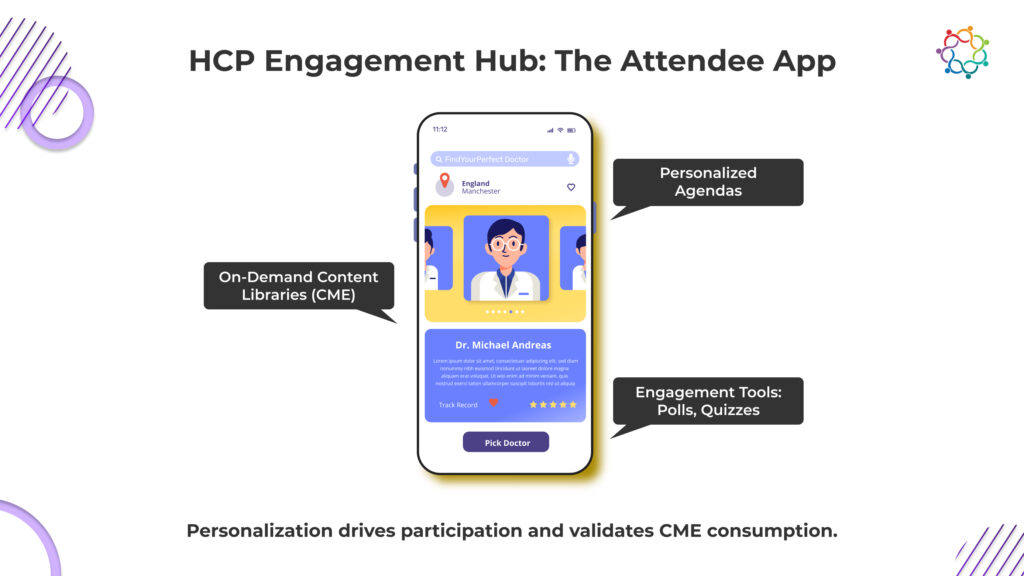
If compliance is at the base, attendee apps serve as the engagement layer connecting physicians, speakers, and organizers in real time.
Today’s pharma events require personalization, and yet personalization has to be conducted in a compliant setting. Attendee apps strike this balance perfectly by giving HCPs curated access to sessions, educational material, and engagement tools without compromising data.
Key Benefits of Attendee Apps:
Customized Agendas: Each doctor can view agendas specific to their specialty, past participation or CME objectives.
On-Demand Libraries of Content: Session recordings, abstracts, and case reports will be available to the attendee post-event for their continued learning.
Interactive Engagement Tools: Engagement in polls, quizzes, and questions during the sessions adds a level of participation and comprehension tracking.
Feedback Loop: Survey the doctor during the session and after the session and feed that into possible analytics dashboards for conference organizers to use to openly discuss and refine future events.
These all move beyond convenience and will enhance retention and knowledge transfer, two important metrics of CME success.
Example:
A leading pharma company in Singapore adopted a mobile event app for its regional CME conference. HCPs could bookmark sessions, participate in live polls, and track their CME progress directly within the app. post-event engagement increased by 40%, and CME completion rates improved significantly.
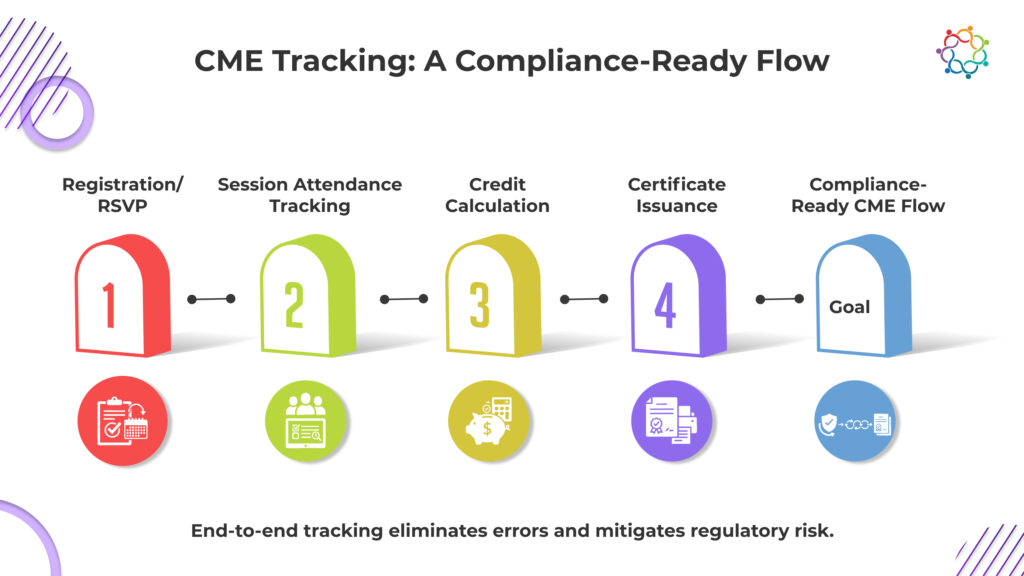
Event management software is what finally brings all moving parts together like registration, attendance, CME tracking, and certification into a single, compliance-ready ecosystem. Disjointed tools are already proving their limitations. As highlighted in Samaaro’s analysis on CME & Medical Conferences, fragmented platforms often lead to lost data, delays, and frustrated attendees.
By contrast, unified event management software offers real-time compliance dashboards, automated credit allocation, and seamless synchronization with medical CRMs ensuring every CME touchpoint is error-free and auditable.
How EMS Powers Compliance and Efficiency:
1. End-to-End Flow: From registration and attendance verification to CME tracking and certificate generation, all within one system.
2. Real-Time Compliance Dashboards: Track CME credits issued, attendance rates, and reporting accuracy live.
3. Automated Certification: No manual data entry, no delays, certificates are automatically issued based on attendance rules.
4. Data Security: Role-based access ensures sensitive HCP data is managed ethically and in compliance with GDPR, HIPAA, or regional equivalents.
5. Automated Reminders: HCPs receive timely alerts for upcoming CME sessions or renewal deadlines, improving retention.
Example:
A worldwide medical equipment organization utilized Samaaro’s EMS and was able to decrease their manual credit reconciliation by 85% while increasing compliance reporting accuracy to 99.7%. The platform also aided the client in automating CME reminders so that physicians completed their milestone of certification cycle on time.
EMS converts an obsolete, paper-intensive process and siloed records into a cohesive automated compliance system.
By the year 2026 the division between medical education and digital engagement will have converged. Doctors require personalization like their experience in consumer technology and regulators require data integrity similar to their experience in clinical systems. Pharmaceutical event technology is developing to accommodate both experiences simultaneously.
Here’s what defines the next generation of pharma events:
AI-Driven Personalization: Intelligent algorithms recommend sessions, content and networking opportunities tailored to each doctor’s specialty and CME rain.
CRM Integration for Medical Associations: Event platforms and medical CRMs can now flow together for ongoing member engagement status to keep accurate member records over time.
Global Compliance Frameworks: Built in tools for region-compliant standards (US, EU, APAC) automates the compliance oversight.
Data-Backed, Insights: Event analytics documenting what sessions drove CME completion and sustained post event engagement post-event.
The future of partnering with pharma for events is data-driven, compliant, personal and calm blending automation with ethical transparency.
Samaaro’s pharmaceutical event technology suite is designed to meet the challenges of the complexities of medical and CME events. The suite integrates compliance management, participant engagement, and data analytics into a single platform.
Essential Features:
Results – CME providers have reported shortened processes to complete certification, lower rates of compliance errors, and higher participant levels of satisfaction when engaging with CME-DCM. To reduce burden on their administration, the solutions offered to allow for better education and engagement outcomes for HCPs.
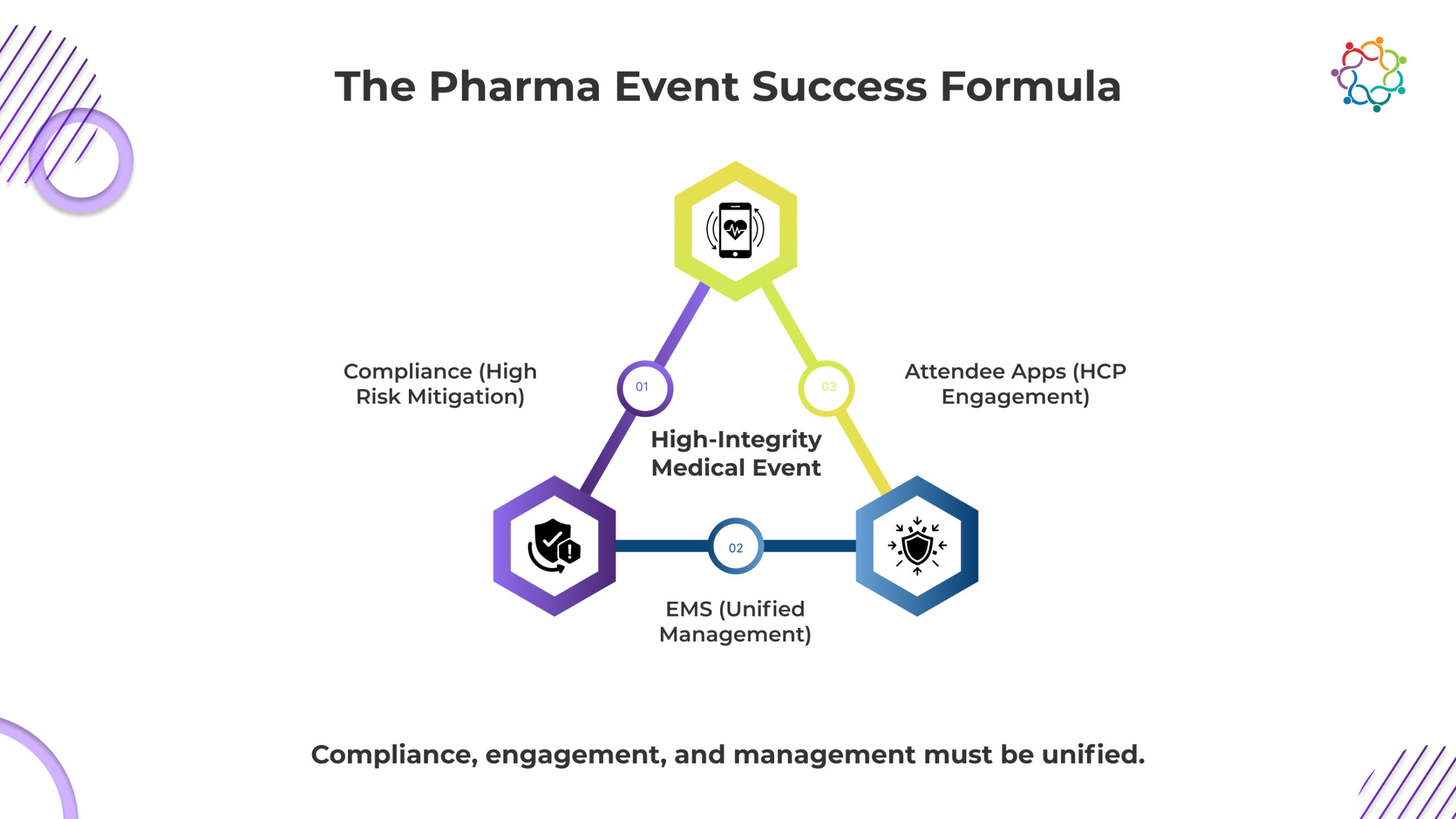
The pharmaceutical industry is changing, and its events need to change with it. Compliance with regulations, attendee engagement, and operational efficiency are no longer separate goals for successful CME and medical meetings but rather interdependent pillars.
If an event does not take compliance seriously, it risks losing its credibility. If it does not have engagement, it risks losing its relevance. To manage both effectively and responsibly, the only possibility is through integrated systems and platforms.
The winning combination of event elements in 2026:

Built for modern marketing teams, Samaaro’s AI-powered event-tech platform helps you run events more efficiently, reduce manual work, engage attendees, capture qualified leads and gain real-time visibility into your events’ performance.
Location


© 2026 — Samaaro. All Rights Reserved.